Arrange the Compounds From Highest to Lowest Boiling Point.
Arrange the following in order of their boiling points from lowest to highest CH3CH2CH3 CH3CHO CH3CH2OH CH34 C CH32 CH CH2 CH3 CH3CH2CH2CH3. Highest boiling point Lowest boiling point Answer Bank CH CHCHOH.
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com
Arrange the compounds by boiling point.
. Solution for Arrange the following in order of their boiling points from lowest to highest CH3CH2CH3. You are currently in a ranking module. Consider how noncovalent interactions would affect the boiling point rather than looking up actual boiling points.
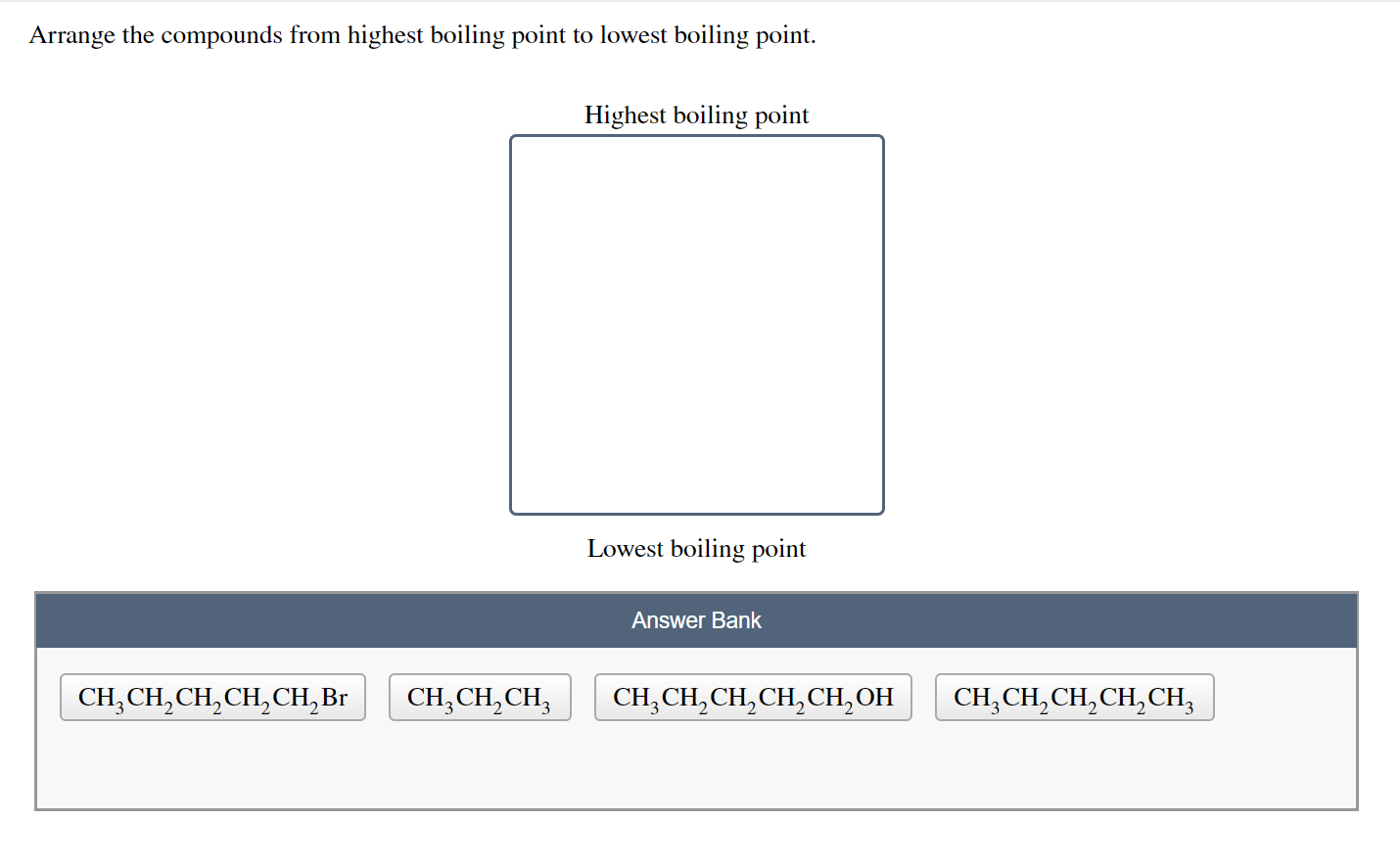
Arrange the following compounds in order of increasing boiling point. Arrange the compounds from lowest boiling point to highest boiling point. Highest boiling point Lowest boiling point CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CH2Br CH3CH2CH2CH2CH3 CH3CH2CH3 Solution.
CH3CCH32CH3 CH3CCH32CH3 position 33 Position 3. Consider how noncovalent interactions would affect the boiling point rather than looking up actual boiling points isopropanolCCl4Dichloromethane1-butenepropane Identify the three true statements -Hydrocarbons exhibit only dispersion forces. CH3CH22OH CH3CH22OH position 22 Position 2.
The order of boiling points is. 1-butene has a higher boiling point than propane because it has higher molecular mass thus greater dispersion forces. Greasy and dirty pot results.
Highest boiling point Lowest boiling point Answer Bank CHCH CHCH. Arrange the compounds from highest boiling point to lowest boiling point. So it is written that lowest 2 highest boiling point.
For the arrangement of compounds above isopropanol has the highest boiling point because it forms hydrogen bonds with water. Arrange the compounds by boiling point pentane. Rank the following from lowest to highest boiling point.
Highest boiling point H₂_. View the full answer. Cellular respiration takes in food and uses it to create atp a chemical which the cell uses for energy.
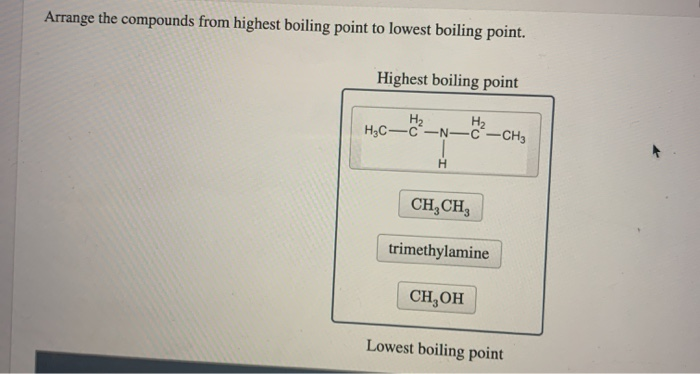
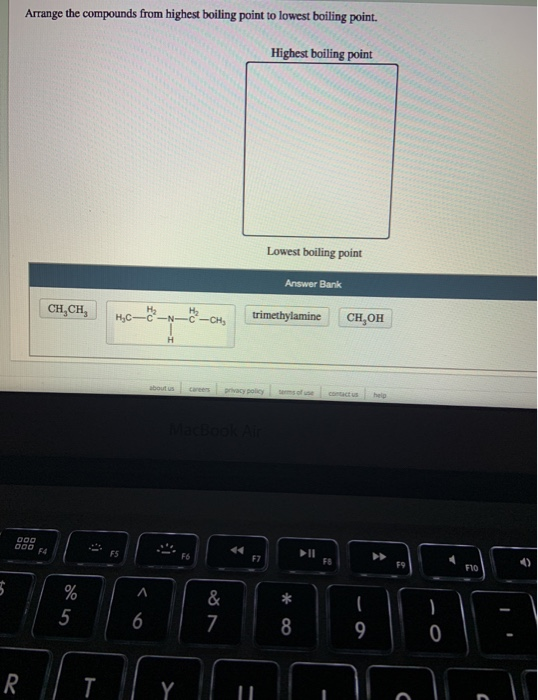
Lowest boiling pointLowest boiling point position 11 Position 1. Highest boiling point Lowest boiling point Answer Bank trimethylamine CH CH OH H2 H3C-N-C-CH3 H. Chemistry questions and answers.
A phrase that functions as a unit. -CCl4 -dichloromethane CH2Cl2 - propane. Arrange the compounds from lowest boiling point to highest boiling point.
CH2Cl2 s molecules are held together by dipole-dipole interactions so its melting point will be between the melting points of CH3OH s and CCl4 s. Solution for Arrange the compounds from highest boiling point to lowest boiling point. For each of the following compounds determine the main intermolecular force.
Please Take care because here it is. Arrange the compounds from highest boiling point to lowest boiling point. Usually this process uses oxygen and is called aerobic respiration.
In this problem Arrange the compounds from lowest boiling 2 highest boiling points. Previous Ich of the following is the correct definition for the term phrasal adverb. But here highest boiling point is on the upper side and on the other side it is lowest boiling point.
It has four stages known as glycolysis link reaction the krebs cycle and the electron transport chain. Turn off browse mode or quick nav Tab to move Space or Enter to pick up Tab to move items between. Clear All CH2CH2CH2CH2CCH Lowest boiling point CHCH2CH2CH2CH2CH2CH3 Intermediate boiling point Highest boiling point CH3CH2CH2CH2CH2CH2OH Previous Next More Questions on General.
A nitrogen LONDON. So here this is actually it is said in the problem. It has only weak London dispersion forces CH_4 has the lowest boiling point.
Ethanol can form hydrogen bonds among themselves. Arrange the compounds from highest boiling point to lowest boiling point. Arrange the compounds in order from highest to lowest boiling point.
Rank these compounds by boiling point from highest to lowest. Clear All CH2CH2CH2CH2CCH Lowest boiling point CHCH2CH2CH. Highest boiling point Lowest boiling point Answer Bank CHCH OH CH CHCHOCHCH.
H2S H2Se H2Te H2Po. Try to do it based on your knowledge of noncovalent interactions without looking up the actual boiling points. Dipole-dipole forces are not as strong as hydrogen bonds so dimethyl ether has a lower boiling point than methanol does.
Arrange the compounds in order from highest to lowest boiling point. Arrange the organic compounds from most soluble in water to least soluble in water. Highest boiling point H₂_.
H3C-CH2-CH2-CH2-CH2-CH3 Lowest boiling point Answer Bank pentane hexane neopentane. Arrange the compounds in order from highest to lowest boiling point. Highest boiling point-hexane-pentane.
Neopentane Hexane Pentane hexane pentane neopentane Arrange the following compounds in order from highest to lowest boiling point. Solution for Arrange the compounds from highest boiling point to lowest boiling point. Arrange the compounds by boiling point.
Dichloromethane possess dipole-dipole interaction which accounts for its higher boiling point. Heptanal is a compound that has seven carbon atoms and an aldehyde functional group is. Diethyl ether can not form hydrogen bonds among themselves but it has a dipole.
Arrange the following compounds in order of increasing boiling point lowest to highest Top label. Finally the C-H bonds in methane are nonpolar so the molecule is also nonpolar. Consider how noncovalent interactions would affect the boiling point rather than looking up actual boiling points.

Solved Arrange The Compounds From Highest Boiling Point To Chegg Com
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com
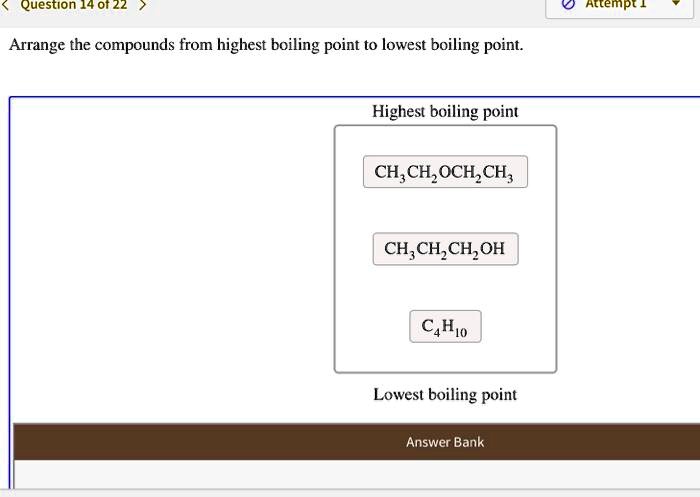
Solved Question 14 01 22 Attempt 1 Arrange The Compounds From Highest Boiling Point To Lowest Boiling Point Highest Boiling Point Ch Ch Och Ch Ch C Ch Jch Oh C4hjo Lowest Boiling Point Answer Bank
No comments for "Arrange the Compounds From Highest to Lowest Boiling Point."
Post a Comment